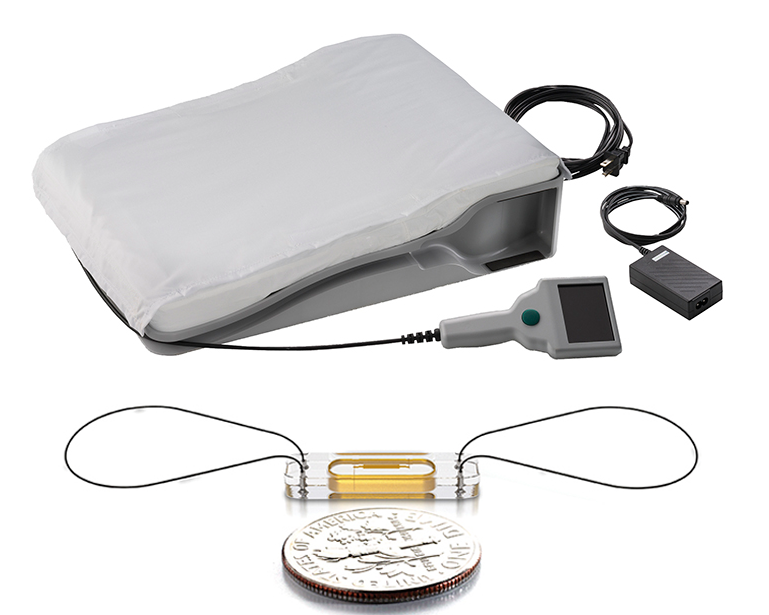
 CardioMEMS is a 15 mm wide by 2 mm deep sealed monitoring sensor (bottom) and a special pillow (top) patients recline on each morning to wirelessly transmit readings to the Heart Care Clinic.
CardioMEMS is a 15 mm wide by 2 mm deep sealed monitoring sensor (bottom) and a special pillow (top) patients recline on each morning to wirelessly transmit readings to the Heart Care Clinic.
Since NKCH began implanting CardioMEMS, an at-home wireless monitoring device for patients with heart failure, three years ago, the hospital has achieved a 54% reduction in HF readmissions.
Not only does the system save patients from additional hospitalizations and the associated costs, but it provides accurate pressure levels in the pulmonary artery for a direct heart pressure assessment.
“This is the single most effective tool to reduce hospitalizations in our heart failure populations. We augment our clinical judgement with the data we gain from the patient’s remote monitor to more accurately provide treatment,” said David Hahn, MD, FACC, an interventional cardiologist with Meritas Health Cardiology. “Patients with heart failure are the most likely to be rehospitalized, but with CardioMEMS they are the least likely to come back.”
The System
The CardioMEMSTM HF System includes a 15 mm wide by 2 mm deep sealed monitoring sensor implanted in the pulmonary artery during a right heart catheterization. Designed to last a lifetime, the sensor does not require a battery or leads. It is powered by radio frequency energy and anchored in the artery wall by two nickel titanium loops. Patients recline on a companion home unit the size of a queen-size pillow to wirelessly transmit their fluid retention and heart pressure readings to the NKCH Heart Care Clinic each morning.
The system is indicated for patients with Class III heart failure who have been hospitalized for HF in the past 12 months, based on New York Heart Association Class guidelines.The device is contraindicated for patients who cannot take dual antiplatelets or anticoagulants for one month after implantation.
Dr. Hahn and Ann Palmer, DNP, FNP-BC, RN, CHFN, review the information on a secure website and contact patients if medication and treatment changes are needed. If pressure readings fall outside prespecified ranges, automated alerts to the Heart Care Clinic are triggered. By measuring subtle PA pressure changes, the team can spot fluid volume increases seven to 10 days before a patient experiences signs or symptoms.
“Because we can track our patients remotely, we can adjust their medications over the phone or bring them in for IV treatments, if needed,” Dr. Hahn added.
The device also allows Dr. Hahn to tailor treatment to the patient’s underlying condition. “It differentiates the causes of shortness of breath, so if a patient with lung disease has bad numbers we know the cause is congestive heart failure. If their numbers are good, we know to look at COPD, a bronchitis exacerbation or another issue. For patients with kidney disease, we can tailor their diuretics more carefully,” Dr. Hahn said.
Research
NKCH is one of only three sites in the Kansas City area participating in the Hemodynamic-GUIDEd Management of Heart Failure six-year clinical trial of up to 3,600 subjects at 140 North American sites. Comprising two arms, the first randomized trial was completed in December 2020 and included Class II, III and IV patients. As part of the trial, patients took part in three study visits in one year, which included completing a 6-minute walk test and an assessment of their quality of life. These were coordinated with the Heart Care Clinic to reduce appointments.
The hospital continues to participate in a nonrandomized arm of only Class III patients. These patients are treated with guideline-based standard of care. “We continue to enroll patients, and they tend do better on this device,” Dr. Hahn said. “They become more vested when they learn their numbers, and they start feeling and breathing better, playing more with their kids, and doing more activities.
Referring physicians may contact Julie Dehner, BSN, RN, at julie.dehner@nkch.org for information about patient enrollment in the GUIDE-HF clinical trial of patients with Class III heart failure.

David Hahn, MD, FACC
Dr. Hahn received his medical degree from the Roy J. and Lucille Carver College of Medicine. After his residency in internal medicine at Vanderbilt University Hospital, he was a fellow in cardiovascular disease at the University of Iowa Hospitals and Clinics.