NKCH is the only hospital in the city conducting two clinical trials of investigational cardiac agents. One is focusing on the efficacy of medication interventions in patients needing urgent surgery or experiencing major bleeding. The other is looking at atrial fibrillation patients needing to restore normal sinus rhythm.
“Patients don’t have to go elsewhere for clinical trials,” said Zafir Hawa, MD, FACC, FSCAI, medical director of cardiac clinical research at NKCH and an interventional cardiologist with Meritas Health Cardiology. “Our patients have the means to participate in advancing science here at NKCH.”
REVERSE-IT
The REVERSE-IT trial is looking at whether bentracimab (PB2452), a recombinant IgG1 monoclonal antibody, provides immediate and sustained reversal of ticagrelor’s antiplatelet effects in patients requiring urgent surgery or experiencing major bleeding.
The arm for those needing urgent surgery has closed, but the second arm for those with major bleeding remains open. The multi-center, open-label, prospective single-arm study is enrolling 200 patients at approximately 200 centers. Those eligible are patients over age 18 with reported use of ticagrelor within the prior three days who require urgent ticagrelor reversal.
In November 2021, the American College of Cardiology reported preliminary results showed bentracimab reverses the effect of ticagrelor, is safe, and is effective in promptly reversing the antiplatelet effect of ticagrelor among patients undergoing urgent surgery/procedure or with major bleeding.
Patients who either needed urgent surgery or a procedure (n = 142) or had major bleeding (n = 8) were enrolled in an open-label single-arm study.
- Total number of enrollees: 150
- Duration of follow-up: 48 hours
- Mean age: 65 years
- Percentage female: 23%
Reversal was noted within five minutes, and the duration of reversal was infusion-time dependent. Effective hemostasis was achieved in most cases, and no rebound increase in platelet activity was noted.
Additionally, during the AHA’s 2021 Scientific Sessions, researchers reported results of the interim analysis, including that “rates of effective hemostasis were adjusted as good or excellent in more than 90% of cases and benefits observed with bentracimab were consistent in all prespecified subgroups, including patients undergoing surgery and with major bleeding.”
“Of the three platelet inhibitors in our arsenal, ticagrelor is the only one with a reversal agent,” Dr. Hawa said. “This gives me peace of mind after our post-ED ticagrelor protocol. This is because we may find the follow-up cardiac catheterization reveals severe coronary artery disease that may require emergent bypass surgery, and we need to reverse the drug’s antiplatelet mechanisms. This is a way to turn off the ticagrelor in as little as 10 minutes and prevent bleeding complications.”
RESTORE-1
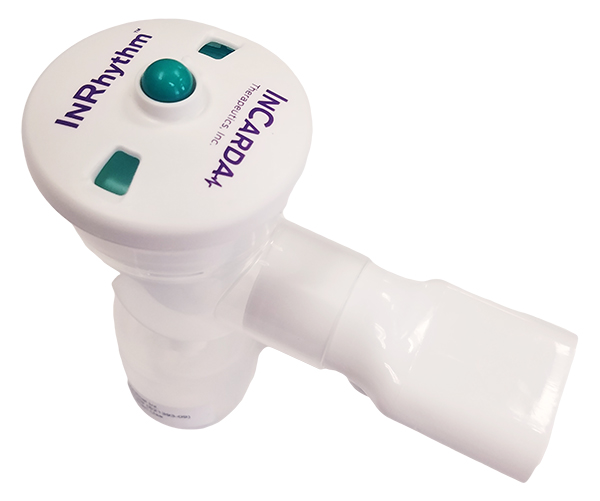
RESTORE-1 looks at if an inhaled version of an antiarrhythmic drug can restore normal sinus rhythm more rapidly than pill form for patients with AFib. Illustration: InCarda Therapeutics
The RESTORE-1 trial seeks to determine if an inhaled version of an anti-arrhythmic drug can restore normal sinus rhythm more rapidly than pill form for patients with atrial fibrillation.
This is a Phase 3, multicenter, randomized, double-blind, placebo-controlled clinical study designed to evaluate the efficacy and safety of FlecIH-103 (flecainide acetate inhalation solution) compared with placebo in patients with recent-onset, symptomatic newly diagnosed or paroxysmal AFib.
Approximately 400 patients are expected to be enrolled. Patients will be randomized 3:1 to receive FlecIH-103 at a total dose of up to 120 mg estimated total lung dose (eTLD) (n=300) or placebo inhalation solution (n=100). Randomization will be stratified by geographic region and duration of symptoms of the current AFib episode (≥1 hour to ≤24 hours and >24 hours to ≤48 hours).
Dr. Hawa noted some patients with AFib are reluctant to take daily medication for their disease. The therapy is being developed as a portable treatment that can be self-administered by patients in any setting to rapidly trigger normal sinus rhythm conversion, as well as for use under medical supervision in a hospital, emergency room or physician office.
“AFib begets AFib, meaning the longer someone is in AFib, the more difficult it is to come out of it,” he said. “If you are in it for five minutes versus 50 minutes, then it’s easier to get out of it. If I can give my patients a medicine that works more quickly than the pill form – so through a nebulizer – the likelihood of their getting out of AFib is going to be higher because a pill takes much longer to be effective.”

Zafir Hawa, MD, FACC, FSCAI
Dr Hawa earned his medical degree from the University of Bombay. He completed his internal medicine residency at the University of North Dakota Medical Center; critical care fellowship at the University of Pittsburgh Medical Center; and cardiology research, cardiology and interventional cardiology fellowships at Western Pennsylvania Hospital.